 |
Department of Engineering |
wxRegSurf is a program for registering pairs of bones and then analysing the distributions of cortical data expressed on the surfaces of these bones. At present, it works with human proximal femurs and L1 vertebrae, but not necessarily other types of bone. wxRegSurf was developed at Cambridge University Engineering Department to first validate, and then exploit, our methods for estimating cortical bone parameters from clinical CT. These methods are fully implemented in the Stradview software package, which is available for download here. If you simply wish to measure cortical parameters on individual femurs or vertebrae, then Stradview is all you need. But if you additionally need to compare these parameters across different scans, or map them onto a canonical morphology for cohort studies, then you will need wxRegSurf as well.
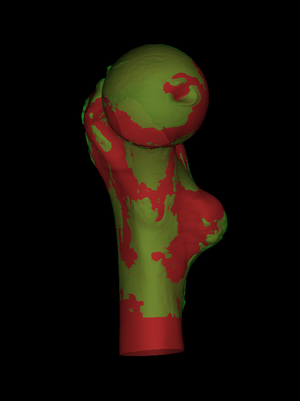 |
wxRegSurf was originally written to help validate our methods for estimating cortical bone parameters from clinical CT. In references [1,2,3,20] (see bibliography below), we describe how we estimate cortical thickness and mass in conventional clinical (low resolution) scans of cadaveric femurs, and then compare them with "gold standard" measurements of the same quantities from high resolution scans. wxRegSurf is used to align the low resolution (red) and high resolution (green) surfaces using a seven degree-of-freedom similarity transformation. Once the surfaces are aligned, wxRegSurf can also assist with comparison of the cortical data distributed across the surfaces. A detailed walk-through of this process can be found here. |
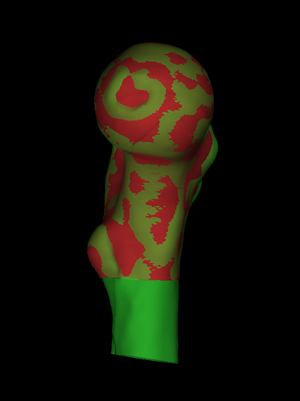 |
Later, we started using cortical bone mapping in a number of cohort studies of the proximal femur [4,5,6,7,8,9,10,11,12,14,17,20]. We were interested in how the distribution of cortical thickness and mass varies between individuals. Our workflow involved statistical parametric mapping (SPM), and we extended wxRegSurf to handle the necessary spatial normalization, whereby each individual bone is first registered onto a canonical morphology. In the screenshot on the left, the green surface is an individual's femur, while the red surface is the canonical femur deformed to match the individual. In this instance, the registration is achieved using a similarity transformation followed by a nonrigid, locally affine deformation. Since wxRegSurf's nomenclature remains unchanged from its early days, the red, canonical surface is still (perhaps confusingly) referred to as the "low resolution" surface, while the green, individual surface is referred to as the "high resolution" surface. It is always the low resolution surface that is deformed to match the high resolution surface. A detailed walk-through of a femur cohort study can be found here. |
 |
Later still, we started exploring cortical parameter estimation on vertebrae, specifically L1 vertebrae [20,24]. We developed a canonical L1 surface and extended wxRegSurf to allow registration of these rather different shapes. In the screenshot on the left, the canonical vertebra (red) has been deformed to match an individual vertebra (green), again using a similarity transformation followed by a nonrigid, locally affine deformation. A detailed walk-through of a vertebra cohort study can be found here. |
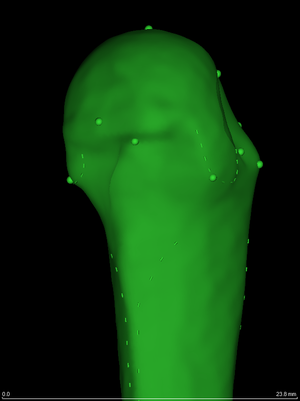 |
One of the registration methods supported by wxRegSurf is the sliding semilandmark approach of geometric morphometrics [13,18]. When point landmarks (spheres, left) and/or curve landmarks (dashes, left) can be identified on the surfaces, wxRegSurf will align them using a thin plate spline, sliding the curve landmarks so as to minimize the bending energy of the spline. A detailed walk-through of a metacarpal study can be found here. While femurs and vertebrae have been the focus of our clinical studies, we have also worked on the cochlea [22,25], the joint space between the femur and the acetabulum [19,21] and at the knee [23], and on other bones in collaboration with palaeoanthropologists [15,16]. Canonical surfaces for a number of small bones in the hand and foot are available on request. |
In addition to these four tutorials, we also provide a quick reference guide to wxRegSurf's user interface.
wxRegSurf was written by Andrew Gee, Chris Bridge and Aya Helmy at Cambridge University Engineering Department. Please direct all enquiries to Andrew Gee. The authors thank Diego Nehab for allowing unrestricted use of his RPly library, which wxRegSurf uses for reading and writing .ply files, and Joe Roberts for his help with the automatic batch file and SSM writers.
G.M. Treece, A.H. Gee, P.M. Mayhew and K.E.S. Poole. High resolution cortical thickness measurement from clinical CT data. Medical Image Analysis, Vol. 14, No. 3, pp. 276-290, June 2010.
G.M. Treece, K.E.S. Poole and A.H. Gee. Imaging the femoral cortex: thickness, density and mass from clinical CT. Medical Image Analysis, Vol. 16, No. 5, pp. 952-965, July 2012.
G.M. Treece and A.H. Gee. Independent measurement of femoral cortical thickness and cortical bone density using clinical CT. Medical Image Analysis, Vol. 20, No. 1, pp. 249-264, February 2015.
K.E.S. Poole, G.M. Treece, G.R. Ridgway, P.M. Mayhew, J. Borggrefe and A.H. Gee. Targeted regeneration of bone in the osteoporotic human femur. PLoS ONE, Vol. 6, No. 1, e16190, January 2011.
K.E.S. Poole, G.M. Treece, P.M. Mayhew, J. Vaculik, P. Dungl, M. Horak, J. Stepan and A.H. Gee. Cortical thickness mapping to identify focal osteoporosis in patients with hip fracture. PLoS ONE, Vol. 7, No. 6, e38466, June 2012.
K.E.S. Poole, G.M. Treece, A.H. Gee, J.P. Brown, M.R. McClung, A. Wang and C. Libanati. Denosumab rapidly increases cortical bone in key locations of the femur: a 3D bone mapping study in women with osteoporosis. Journal of Bone and Mineral Research, Vol. 30, No. 1, pp. 46-54, January 2015.
T. Whitmarsh, G.M. Treece, A.H. Gee and K.E.S. Poole. Mapping bone changes at the proximal femoral cortex of postmenopausal women in response to alendronate and teriparatide alone, combined or sequentially. Journal of Bone and Mineral Research, Vol. 30, No. 7, pp. 1309-1318, July 2015.
S.J. Allison, K.E.S. Poole, G.M. Treece, A.H. Gee, C. Tonkin, W.J. Rennie, J.P. Folland, G.D. Summers and K. Brooke-Wavell. The influence of high impact exercise on cortical and trabecular bone mineral content and 3D distribution across the proximal femur in older men: a randomised controlled unilateral intervention. Journal of Bone and Mineral Research, Vol. 30, No. 9, pp. 1709-1716, September 2015.
G.M. Treece, A.H. Gee, C. Tonkin, S.K. Ewing, P.M. Cawthon, D.M. Black and K.E.S. Poole. Predicting hip fracture type with Cortical Bone Mapping (CBM) in the osteoporotic fractures in men (MrOS) study. Journal of Bone and Mineral Research, Vol. 30, No, 11, pp. 2067-2077, November 2015.
A.H. Gee, G.M. Treece, C. Tonkin, D.M. Black and K.E.S. Poole. Association between femur size and a focal defect of the superior femoral neck. Bone, Vol. 81, pp. 60-66, December 2015.
T. Whitmarsh, G.M. Treece, A.H. Gee and K.E.S. Poole. The effects on the femoral cortex of a 24 month treatment compared to an 18 month treatment with teriparatide: a multi-trial retrospective analysis. PLoS ONE, Vol. 11, No. 2, e0147722, February 2016.
T.D. Turmezei, G.M. Treece, A.H. Gee, A.F. Fotiadou and K.E.S. Poole. Quantitative 3D analysis of bone in hip osteoarthritis using clinical computed tomography. European Radiology, Vol. 26, No. 7, pp. 2047-2054, July 2016.
P. Gunz, P. Mitteroecker and F.L. Bookstein. Semilandmarks in three dimensions. In D.E. Slice (editor), Modern Morphometrics in Physical Anthropology, pp. 73-98, Springer US, 2005.
K.E. Poole, L. Skingle, A.H. Gee, T.D. Turmezei, F. Johannesdottir, K. Blesic, C. Rose, M. Vindlacheruvu, S. Donell, J. Vaculik, P. Dungl, M. Horak, J.J. Stepan, J. Reeve and G.M. Treece. Focal osteoporotic defects play a key role in hip fracture. Bone, 94:124-134, January 2017.
Z.J. Tsegai, M.M. Skinner, A.H. Gee, D.H. Pahr, G.M. Treece, J-J. Hublin and T.L. Kivell. Trabecular and cortical bone structure of the talus and distal tibia in Pan and Homo. American Journal of Physical Anthropology, 163(4):784-805, August 2017.
Z.J. Tsegai, N.B. Stephens, G.M. Treece, M.M. Skinner, T.L. Kivell and A.H. Gee. Cortical bone mapping: an application to hand and foot bones in hominoids. Comptes Rendus Palevol, 16(5-6):690-701, August-September 2017.
T. Whitmarsh, G.M. Treece, A.H. Gee and K.E.S. Poole. An exploratory study into measuring the cortical bone thickness from CT in the presence of metal implants. International Journal of Computer Assisted Radiology and Surgery, 12(12):2079-2086, December 2017.
A.H. Gee, G.M. Treece and K.E.S. Poole. How does the femoral cortex depend on bone shape? A methodology for the joint analysis of surface texture and shape. Medical Image Analysis, 45:55-67, April 2018.
T.D. Turmezei, G.M. Treece, A.H. Gee, R. Houlden and K.E.S. Poole. A new quantitative 3D approach to imaging of structural joint disease. Scientific Reports 8, 9280, June 2018.
G.M. Treece and A.H. Gee. Cortical bone mapping: measurement and statistical analysis of localised skeletal changes. Current Osteoporosis Reports, 16(5):617-625, October 2018.
T.D. Turmezei, G.M. Treece, A.H. Gee, S. Sigurdsson, H. Jonsson, T. Aspelund, V. Gudnason. and K.E.S. Poole. Quantitative 3D imaging parameters improve prediction of hip osteoarthritis outcome. Scientific Reports 10, 4127, March 2020.
A.H. Gee, Y. Zhao, G.M. Treece and M.L. Bance. Practicable assessment of cochlear size and shape from clinical CT images. Scientific Reports 11, 3448, February 2021.
T.D. Turmezei, S.B. Low, S. Rupret, G.M. Treece, A.H. Gee, J.W. MacKay, J.A. Lynch, K.E.S. Poole and N.A. Segal. Quantitative three-dimensional assessment of knee joint space width from weight-bearing CT. Radiology, 299(3):649-659, June 2021.
K.E.S. Poole, G.M. Treece, R.A. Pearson, A.H. Gee, M.A. Bolognese, J.P. Brown, S. Goemaere, A. Grauer, D.A. Hanley, C. Mautalen, C. Recknor, Y.C. Yang, M. Rojeski, C. Libanati and T. Whitmarsh. Romosozumab enhances vertebral bone structure in women with low bone density. Journal of Bone and Mineral Research, 37(2):256-264, February 2022.
F. Hrncirik, I.V. Roberts, C. Swords, P.J. Christopher, A. Chhabu, A.H. Gee and M.L. Bance. Impact of scala tympani geometry on insertion forces during implantation. Biosensors 12(11):999, November 2022.